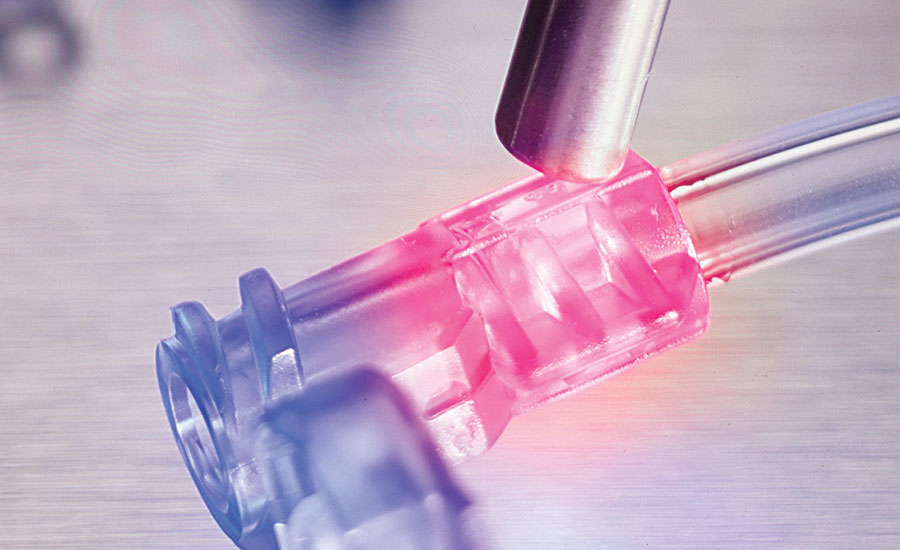
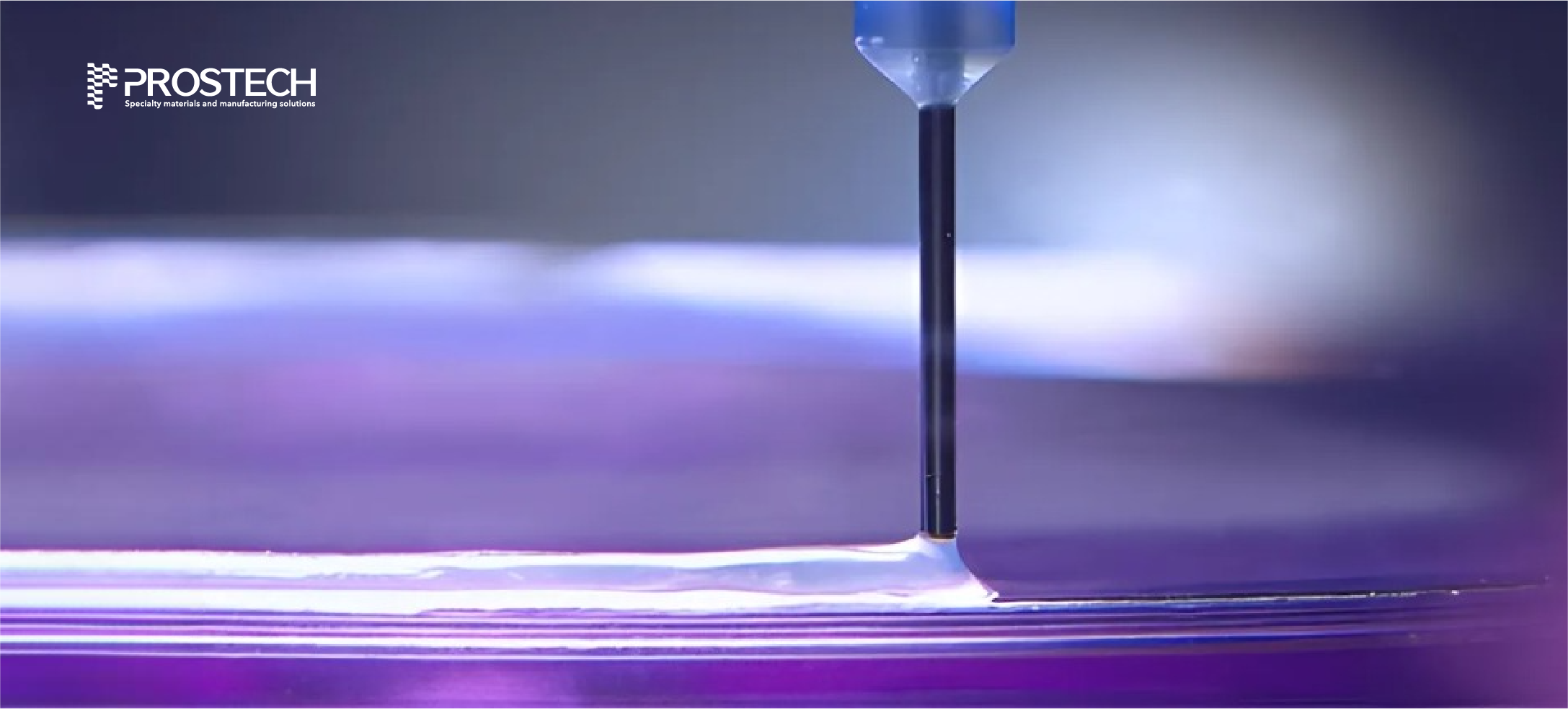
MARKET CHALLENGES

Hard To Bond Substrates Nylon, Coc/cop And Peba

Biocompatibility Testing

Medical-grade Standards Requirement

The Impact Of Sterilization Processes On Bonding Joint
PROSTECH’S SOLUTIONS

In conjunction with the development of Medical Technology, Medical Devices are increasingly diverse. They range in scope from a Q-tip to an X-ray machine and the manufacturing process involves uncountable applications. PROSTECH is confident to keep pace with medical technology development and provide customers with the utmost medical adhesive solutions.
Firstly, our range of adhesives for medical devices is equally as diverse and has been carefully selected to demonstrate our capabilities. There are a few key features of the popular products. However, the effectiveness we mentioned will be reflected in our customizing product line which can be formulated to best suit your requirements.
Furthermore, we do not promise to provide the best adhesive Adequate Adhesive Solution. Some medical device adhesive applications require adhesives formulated to pass biocompatibility testing (not all medical devices), others require structural bonding of equipment. It is more important to select the right adhesive rather than the best one.
In Medical Devices Manufacturing Industry, whether you are seeking a unique UV curable adhesive to bond a state-of-the-art oxygenator or a structural bond of an MRI housing we are here to help.

Bonding solutions for hard to bond substrates (Nylon, COC/COP and PEBA)

Biocompatibility adhesives

Medical-grade standards adhesives

Sterilization Resistant Adhesives
MEDICAL-GRADE STANDARDS

ISO 10993 biocompatibility standards (ISO 10993-5,-10,-11,-4,-6)
Depending on the application requiring an adhesive solution, many of our products also have passed ISO 10993 testing for skin sensitization and irritation as well.

ISO 13485 Medical Devices Quality Management
Ensuring that our products are made consistently and that they will conform to Medical Device Quality Management Standards.

USP Class VI Bio-compatibility of Materials
set by the US Pharma Convention (USP) with a focus on medications, healthcare technologies, food ingredients, and materials used in medical devices.

FDA Approval for Medical Devices
Since the user must submit his part for final testing and FDA certification, an adhesive’s qualification to ISO 10993 or USP Class VI is viewed as sufficient to allow a user to determine “probable” device acceptance.
WHY CHOOSE PROSTECH

Customer Sample Testing Support

Global Delivery

Medical-grade Adhesives Available For Shipping

Automate And Optimize Production Process
MEDICAL-GRADE ADHESIVE PRODUCTS
Detailed Application



Useful Information
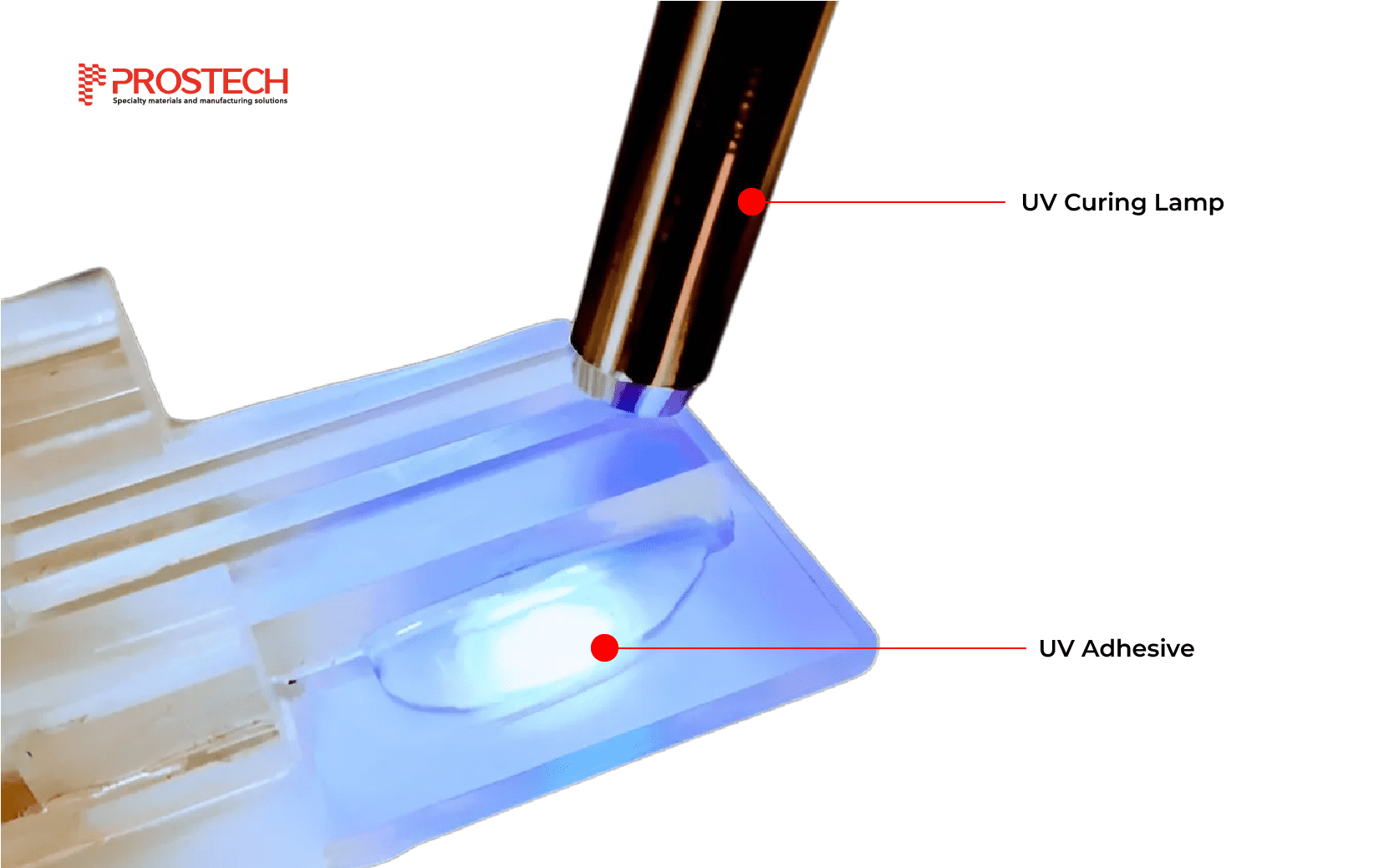
UV-curable cyanoacrylate adhesives (UVCA) for medical devices
When it comes to medical device manufacturing, adhesive technologies play a pivotal role in the efficient assembly and functionality of
7 Reasons why Light-Curable Adhesives are Ideal for Medical Device Assembly
As in-home patient care becomes more common, material regulations tighten, and the demand for safer, less invasive devices increases, selecting
How to bond Hard-to-bond Substrates (Nylon, COC/COP and PEBA) in Medical Devices Assembly?
In medical device assembly, substrates such as Nylon, COC/COP (cyclic olefin copolymer/polymer), and PEBA (polyether block amide) are favored for
Solvent Welding and Ultrasonic Welding Alternatives in Medical Assembly
When assembling medical devices, ultrasonic welding and solvent welding are popular joining methods for plastic parts. Although they offer fast processing and strong
What is UV Adhesive? Applications of UV Adhesive in Medical Industry
Choosing the right adhesive can be a complex decision that requires balancing the demands of production processes with the performance
UV Adhesive: A guide to overcome all the challenges for optimizing its benefits
UV adhesive is a common type of adhesive in use today, employed across various manufacturing industries due to its superior
What are the advantages of using UV Adhesive?
UV adhesives have a fascinating history that dates back to the 1960s when traditional adhesives often required a very long
How to prevent blooming issue of Instant Adhesive or Cyanoacrylate Adhesive
The cause and the nature of blooming in CA adhesive Cyanoacrylates (CAs) are commonly known as instant adhesive or super
The 9 Myths about Cyanoacrylate Adhesives
Cyanoacrylate adhesives (CA adhesives) — also called “super glues” or instant adhesives — are popular in many industrial and domestic
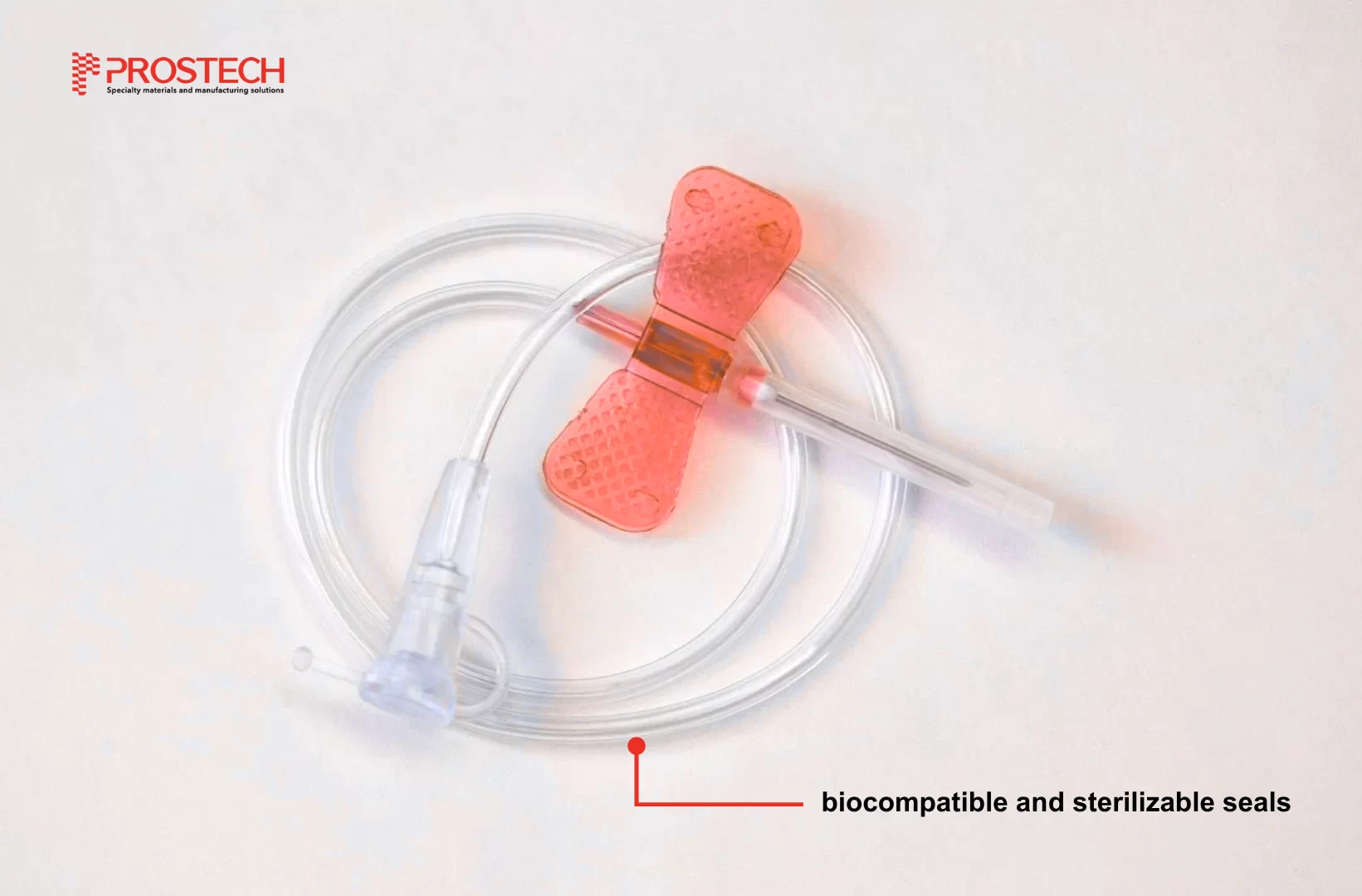
Guideline to disposable medical devices adhesives
Disposable medical device assemblies require adhesives with strong, reliable, biocompatible medical liquid and sterilization-resistant properties. Adhesives for any application should
ACRYLIC ADHESIVES USES IN INDUSTRIAL MANUFACTURING
Structural Acrylic Adhesives are characterized by their high bond strength and wide range of material compatibility. Their applications are in
Is Your Adhesive Biocompatible?
A biocompatible adhesive is designed for safe use in medical applications involving contact with biological tissues or fluids. These adhesives